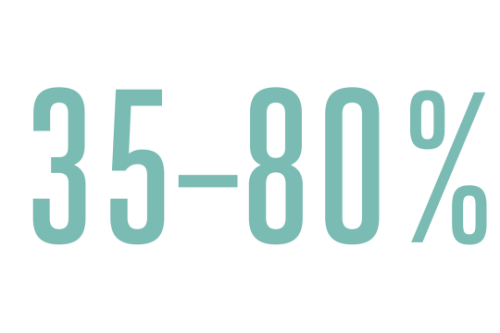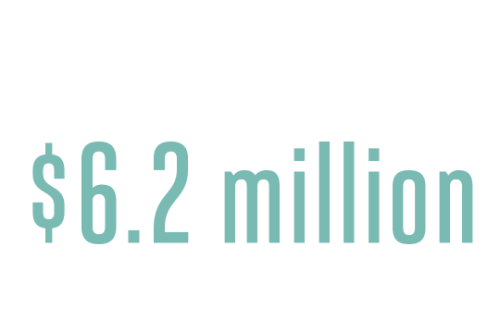Reuniting Babies and Their Bottles
By Lauren Ingeno | Illustration: John McGlasson, BA '00, MFA '03
Monica Jennings could not swallow when she was born prematurely in 1994 at Boston’s Brigham and Women’s Hospital. She would aspirate liquids, inhaling them into her lungs, which led to a series of pneumonia and sinus infections. Soon after her birth, doctors inserted a tube into her nose to feed her, and at three weeks, put another in her trachea to protect her airway. Though they told her mother, Lisa, that the tubes likely would be temporary, they remained for more than a decade.
Ms. Jennings spent the first months of her daughter’s life consulting geneticists, neurologists, speech therapists and gastroenterologists, but no one could tell her why Monica was unable to swallow. For two years, they called her symptoms a medical mystery.
“My sister and I lived together, and my nephew was born a few weeks after Monica. So I came home every night to this very healthy, very hungry, vocal baby. And I had exactly the opposite,” Ms. Jennings says. “I couldn’t feed her or hold her. Oh, it was brutal. Brutal.”
Doctors finally diagnosed Monica with 22q11.2 deletion syndrome, known as 22Q or DiGeorge syndrome, a disorder that affects an estimated 1 in 4,000 people, though it may be more, as experts suspect it is underdiagnosed. DiGeorge syndrome is caused by a small amount of genetic material missing on the long arm of chromosome 22. Most often, the deletion occurs at random and is rarely inherited from a parent.
The syndrome can lead to an extraordinarily large and diverse range of health and cognitive issues, from learning disabilities and language delays to heart defects and seizures, making it difficult to recognize.
But one of the most debilitating symptoms of DiGeorge, and the root of overwhelming anxiety for parents like Ms. Jennings, is the inability to properly chew, swallow and digest food.
Dysphagia, the medical term broadly applied to these symptoms, is a dangerous complication that affects not only DiGeorge patients but also at least one-third of those with neurodevelopmental disorders, like Down syndrome and autism.
“It must be terrible, to be brand new in the world and every time someone comes at you with food it hurts,” says Anthony LaMantia, a professor of pharmacology and physiology in the School of Medicine and Health Sciences and director of GW’s Institute for Neuroscience.
And for patients like now 21-year-old Monica, it is an enduring battle. Though she first ate solid food—a banana—at age 13, Monica struggles daily with dysphagia and the health issues that stem from it.
"I thought that if you were going to solve a compelling problem, this would be it"
Anthony LaMantia
(Photo: William Atkins)
Dr. LaMantia has been studying DiGeorge syndrome for more than a decade. He has primarily focused on disruptions in the development of the cerebral cortex, the part of the brain that does the heavy lifting of memory, learning and cognition. However, as he talked to pediatricians, he realized they were spending a frustrating amount of time trying—to little avail—to relieve swallowing problems in DiGeorge patients.
“None of the clinical literature addressed, ‘Why is this happening? And what can you do to fix it?’ I thought that if you were going to solve a compelling problem, this would be it,” Dr. LaMantia says.
In a 2013 study, Dr. LaMantia and a team of GW researchers reported finding that an existing, genetically modified 22Q mouse model exhibited all the major dysphagia symptoms found in DiGeorge patients—including issues with weight gain, swallowing and lung infections—opening the door to a more detailed look at the disruptions underyling the problem.
Analyzing the mouse model, the team found that issues with eating and swallowing were directly linked to a disruption in the embryonic development of cranial nerves—a dozen pairs of nerves that originate in the brain and carry out functions related to different senses in the body. The discovery reversed a common assumption that dysphagia symptoms arise after a child is born.
“It turns out that in the development of the earliest, prenatal steps that set up craniofacial structures—like the mouth and jaw—as well as the brain structures and nerves that control those muscles, something just isn’t quite right,” says study co-author Tom Maynard, an associate research professor of pharmacology and physiology.
Now, after three years of preliminary studies, Dr. LaMantia has assembled an interdisciplinary team of researchers from GW and Children’s National Health System that will use the mouse model to understand how and why early brain disruptions lead to dysphagia in patients with developmental disorders.
The three-part project is funded by a $6.2 million grant from the National Institute of Child Health and Human Development and brings together a group comprising neuroscientists, geneticists, developmental biologists and clinicians. Along the way, the team will also consult with pediatricians and speech therapists at Children’s National to see how they may be able to translate their research findings into clinical practice.
Prevalence of DiGeorge syndrome, though experts suspect it is underdiagnosed.
Estimated percentage of people with neurodevelopmental disorders who are affected by dysphagia (difficulty eating).
Amount of new grant from the National Institute of Child Health and Human Development grant that brings together the efforts of seven GW faculty members.
The research promises not only to define new therapies and prevention strategies that may improve the lives of those with DiGeorge syndrome but may also have an impact far beyond those patients.
“If we can understand how the neural circuits are compromised in the part of the brain that regulates this very simple, but very essential behavior of swallowing and food indigestion,” Dr. LaMantia says, “that may give us insight and a framework about how neural circuits are compromised for much more complex behaviors that go awry in developmental disorders.”
The Broken Blueprint
Though it seems like a simple, even automatic task, the act of eating is a complex orchestration of brain and body. When all goes according to plan, food is chewed, mixed with saliva and positioned on the tongue for transport to the back of the mouth. Sensory receptors in the tongue and throat trigger the swallow, and the palate rises and closes to prevent food from entering the nasal cavity. The voice box elevates to protect the airway, and food is routed into the throat.
“There is a whole process of preparing the food, directing it and keeping it on the right path,” says Dr.
Maynard, who studies cell signaling during neural development and will serve as co-investigator in two of the dysphagia project studies. “It actually takes fairly fine motor control, considering that most of us don’t have to think about it.”
In DiGeorge patients, various points in that sequence are broken. Doctors can surgically correct severe facial defects, such as cleft palate, in an attempt to alleviate dysphagia. But many children with developmental disorders who aspirate do not have any visible facial abnormalities. This suggests something is going haywire in the brain, rather than in their facial mechanics.
“As soon as any neural mechanism is involved, it becomes a much harder problem,” Dr. LaMantia says. “You can recognize it clinically, but the underlying brain control of this behavior, and also the peripheral mechanism that must be put in place, is very complicated. Our ability to really fix it has been limited, because our knowledge has been limited.”
The mystery mirrors cases of children with developmental disorders who have issues with eye alignment, which can lead to double vision. For years, doctors tried to correct these problems—often unsuccessfully—by operating on eye muscles. But the root of the issue was deeper.
“The surgery wasn’t dealing with the problem,” Dr. LaMantia says. “The problem was in the cranial nerve circuits in the brain stem that control eye movement.”
The findings inspired Dr. LaMantia, who thought that those same disruptions could be causing dysphagia in DiGeorge patients.
The pattern of genes that are switched on and off—called gene expression—in the embryonic brain stem lays out the blueprint for the proper development of the face, mouth, lips and jaw. It also gives rise to the nerve cells that control feeding and swallowing. While analyzing the brain stems of the 22Q mutant mouse models, Dr. LaMantia and his team discovered that gene expression levels and patterning in this region were highly disorganized. The brain’s instruction booklet wasn’t providing correct information to the face.
“We were able to show in the animal model that in a surprisingly classical, molecularly mechanistic way, the initial formation of that part of the brain was disrupted,” Dr. LaMantia says. “What we now have to figure out is what the consequence of that disruption is.”
In their new project, Dr. LaMantia, associate director Sally Moody, a professor of anatomy and
regenerative biology and an expert in craniofacial development, and Norman Lee, a professor of pharmacology and physiology who specializes in genomics, will investigate how these early interruptions establish changes in neural circuits in feeding and swallowing. They’ll see how neurons in the brain stem develop and migrate during the prenatal period and what factors may cause that migration.
Simultaneously, David Mendelowitz, vice chair of the Department of Pharmacology and Physiology, will lead a study into whether the neurons in the brain that control feeding and swallowing may be misfiring in DiGeorge patients. He will work with Dr. Maynard, Dr. Lee and Anastas Popratiloff, director of GW’s Center for Microscopy and Image Analysis, in these efforts.
“The question is, what’s causing the swallowing difficulty? Is it the function of the individual motor neurons? Is it the sequence of events? Is it the timing or the magnitude of these changes?” Dr. Mendelowitz says.
By identifying the receptors or neurotransmitters that may be overactive or underactive in the brains of DiGeorge patients, Dr. Mendelowitz is hoping to point the way to targeted therapies for improving dysfunctional swallowing.
From the various arms of the project, the team also hopes to better understand the wide variability of DiGeorge syndrome’s kaleidoscope of symptoms. In the mouse model, the researchers saw remarkable variation among siblings, which should have nearly identical genetic makeups. One goal, Dr. LaMantia says, will be to identify other genes at the root of dysphagia, in order to create a “genetic map of vulnerabilities” that could be used to predict or diagnose physical problems.
A Dietary Fix
As the project got under way this spring, the researchers came into it with one idea for straightening out the circuitry that goes off course.
Retinoic acid, the active form of vitamin A, is instrumental in the patterning of nerves that initiate swallowing. The researchers’ 2013 study found that the 22Q mouse embryos appear to be hypersensitive to even the smallest changes of the nutrient.
“Really high or low doses of retinoic acid can change those cranial nerves, so it makes sense that it would cause swallowing defects,” says Irene Zohn, an associate professor of pediatrics at GW and a researcher at Children’s National. “But it seems that the mice carrying the 22q11.2 deletion are not able to compensate for small ups and downs.”
Dr. Zohn, a developmental biologist, will lead efforts to determine whether modifying the vitamin A intake in mouse model mothers could prevent dysphagia in their offspring.
“Our preliminary data shows that yes, things can change,” she says. “But the question is: How much do all these changes come together to affect the physical abnormality?”
If the researchers do find a link between vitamin A intake and the emergence of dysphagia in people with DiGeorge syndrome, clinicians may be able to offer corrective dietary guidelines for expectant mothers.
Within the next five years, the researchers hope to get closer to assembling the pieces that make up the complex picture of pediatric dysphagia and, in turn, uncovering new ways to prevent and treat the condition.
In the meantime, nearly two decades after Monica Jennings was diagnosed with DiGeorge syndrome, her mother’s search for answers continues, as well. Years of medications, therapy and multiple surgeries have yet to resolve her daughter’s difficulties with swallowing.
“I could go on for ages about the paths we have followed, plowed or dismissed, and the things we’ve discovered along the way,” Lisa Jennings says.
New research endeavors give her hope, though she also understands better than most the challenges facing scientists. “I have an entirely different respect for the complexity of human biology,” she says, “and just how fragile the body can be.”
Other Summer Features
Five Years Later in Haiti
Half a decade after a catastrophic earthquake, a Pulizter Prize winner finds that life in the beleaguered nation goes on, as it always has.
Promise Keeper
MacArthur Fellow Jonathan Rapping is helping the government fulfill its duty to stand up for the indigent accused with his program, Gideon's Promise.
"We Are Always Activists First"
Students on the front lines of a national movement against sexual violence find a battlefield with no boundaries.